![Battery acid is 4.22 M aqueous H2SO4 solution, and has density of 1.21 g cm ^- 3 . What is the molality of H2SO4 ? [H = 1, S = 32, O = 16] Battery acid is 4.22 M aqueous H2SO4 solution, and has density of 1.21 g cm ^- 3 . What is the molality of H2SO4 ? [H = 1, S = 32, O = 16]](https://d1hj4to4g9ba46.cloudfront.net/questions/1356160_1287105_ans_848c804ee73c44c395503b2d13066dbb.jpg)
Battery acid is 4.22 M aqueous H2SO4 solution, and has density of 1.21 g cm ^- 3 . What is the molality of H2SO4 ? [H = 1, S = 32, O = 16]

Converting mass percent to molarity: the density of a 24.5 mass % solution of sulphuric acid ` - YouTube

Effects of sulfuric acid molarity on HPR with electrolyte solutions... | Download Scientific Diagram

What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora

SOLVED: Using the average molarity 3.14 M of the sulfuric acid solution, calculate the mass percent of sulfuric acid in your unknown solution. Assume the density of your solution is close to

SOLVED: called battery acid because It" used In car batterles It Is a very strong acid that can cause Sulfuric add Is often very serious chemical burns upon contact: It can lead

What is the molality of a solution containing 200 mg of urea (molar mass 60 g mol^-1 ) dissolved in 40 g of water?
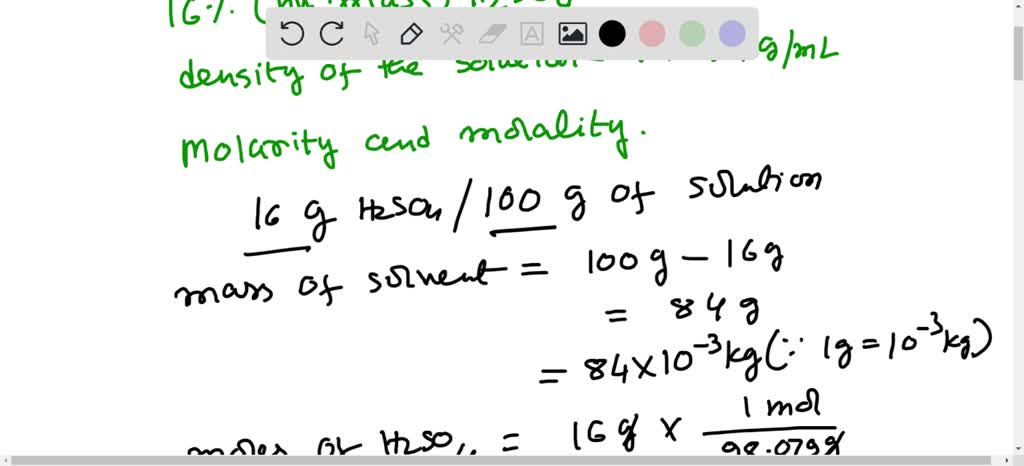
SOLVED: Please help me solve for this, thank you very much. Stay safe Sulfuric acid is also known as battery acid with a Molecular Formula H2SO4 and Molar Mass of 98 grams/mole
Battery acid is 4.27M H2SO4(aq) and has density 1.25 g/ML. What is the molality of H2SO4 in the solution?

:max_bytes(150000):strip_icc()/car-battery-recycling-container-with-warning-notices-battery-acid-flusco-household-waste-recycling-centre-cumbria-uk-121814398-57a4e5055f9b58974a7355d8.jpg)